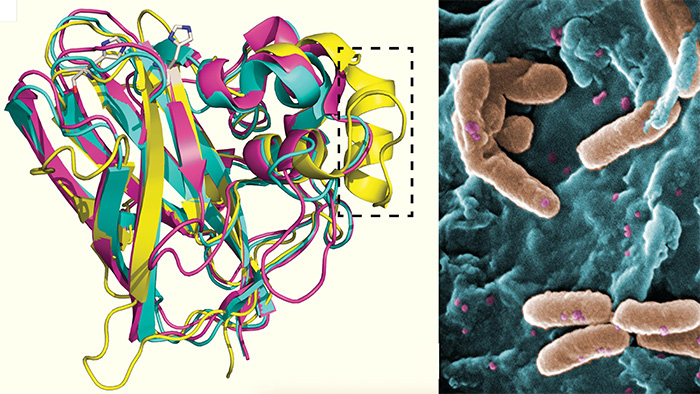
UC San Diego researchers determined the structure of Pseudomonas aeruginosa CbpD protein by X-ray crystallography and Alphafold2 machine learning prediction (left). P. aeruginosa bacteria attaching to epithelial cells (right). Source: Wikipedia
Pseudomonas aeruginosa is a common bacterial pathogen that causes serious and sometimes life-threatening infections in the blood, lungs and other parts of the body. It is particularly well-known in hospital settings, where it infects tens of thousands of hospitalized or immunocompromised patients in the United States each year, including those with cystic fibrosis lung disease or undergoing cancer chemotherapy.
Scientists are constantly on the lookout for new ways to protect against bacterial infections like P. aeruginosa, which are prone to developing antibiotic resistance. In a new study published on July 17, 2023 in the Proceedings of the National Academy of Sciences (PNAS), UC San Diego researchers found hope in an unexpected place. In collaboration with colleagues in Norway, the team showed that an unusual class of enzymes, lytic polysaccharide monooxygenases (LPMOs), could be used as a vaccine to protect against P. aeruginosa infection.
LPMOs are powerful copper enzymes that cleave polysaccharides (long chains of sugar molecules bonded together) such as cellulose, found in the cell walls of plants, or chitin, found in crab shells. They do this through oxidative reactions, in which electrons work with oxygen or peroxide to dissolve the chemical bonds between sugars — a process that contributes to the recycling of carbon in nature.
Since their discovery in 2010, LPMOs have fascinated biochemists for their unique biochemical mechanics and their potential industrial applications in biomass conversion (using biological waste to produce renewable energy). But their potential for clinical use had yet to be realized — until now.
In 2019, Fatemeh Askarian, PhD, an assistant project scientist at UC San Diego School of Medicine, discovered that P. aeruginosa produced an LPMO called chitin binding protein D (CbpD), and this protein was required for the bacteria to produce disease. Askarian immediately considered the medical significance of this unexpected finding, knowing the threat this particular bacteria posed on hospital patients.
These results inspired Askarian and other team members in the laboratory of Victor Nizet, MD, Distinguished Professor of Pediatrics and Pharmacy at UC San Diego, to explore whether the CpbD protein could be purified and used as a vaccine to defend against serious P. aeruginosa infection.
In the new study, the researchers used X-ray crystallography and machine learning algorithms to identify the optimal immune epitopes (the part of an antigen that is recognized by the immune system) for enhanced vaccine performance. They then showed that the full-length CbpD protein and specific subdomains could be used to immunize mice and protect them against lethal pneumonia or sepsis.
The research demonstrates that CbpD helps P. aeruginosa avoid clearance from lung tissues and the blood, but that stimulating the production of antibodies to neutralize CbpD helps the host immune system regain the upper hand.
Since LPMOs are found in several additional important human bacterial pathogens, the researchers are now expanding their study of these curious enzymes in host-pathogen interactions and as potential therapeutic targets.
Read the full paper here: https://www.pnas.org/doi/10.1073/pnas.2301538120
– Nicole Mlynaryk